|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Welcome
to the dissolved oxygen (D.O.) theory Page. Dissolved oxygen
from A to
Z Dissolved Oxygen:
The air we breathe contains about 20% oxygen. Fish and other aquatic organisms require oxygen as well. The term Dissolved Oxygen (DO or D.O.) refers to the amount of free oxygen dissolved in water which is readily available to respiring aquatic organisms. State water quality standards often express minimum concentrations of dissolved oxygen which must be maintained in order to support life as well as be of beneficial use. Levels of dissolved oxygen below 4-5 milligrams per liter affect fish health and levels below 2 milligrams per liter can be lethal to fish.
Additionally, biochemical oxygen demand (BOD) is commonly used with reference to effluent discharges and is a common, environmental procedure for determining the extent to which oxygen within a sample can support microbial life. The test for BOD is especially important in waste water treatment, food manufacturing, and filtration facilities where the concentration is crucial to the overall process and end products. High concentrations of DO predict that oxygen uptake by microorganisms is low along with the required break down of nutrient sources in the medium.
Basic
principles of Polagrography cell Liquid
and Air state of equilibrium is reached when the partial pressure of
oxygen, i.e. the part of the total pressure that is due to oxygen, is
equal in air and in liquid. The liquid is then saturated with oxygen.
Figure
1.1 Air and liquid oxygen equilibrium
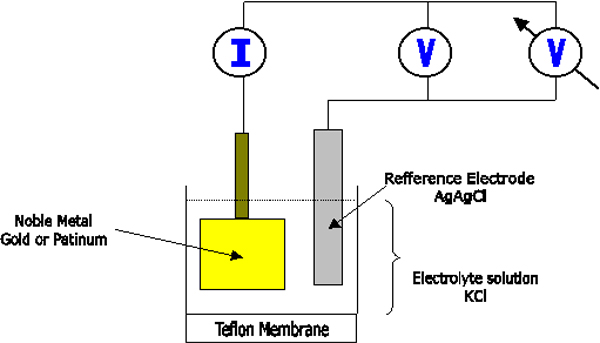
When
an electrode of noble metal such as platinum or gold is made 0.6 to 0.8 V
negative with respect to a suitable reference electrode such as AgAgCl or
an calomel electrode in a neutral KCI solution (see Figure 1.2), the
oxygen dissolved in the liquid is reduce at the surface of the noble
metal.
Figure
1.2 Polarographhy diagram
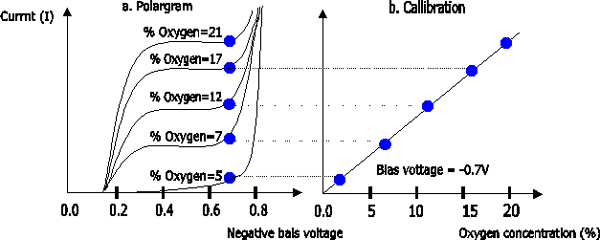
This
above phenomenon can be observed from a current to voltage diagram called a
polarogram of the electrode. As shown in Figure 1.3a, the negative voltage
applied to the noble metal electrode (called the cathode) is increased,
the current increases initially but soon it becomes saturated. In this
plateau region of the polarogram, the reaction of oxygen at the cathode is
so fast that the rate of reaction is limited by the diffusion of oxygen to
the cathode surface. When the negative bias voltage is further increased,
the current output of the electrode increases rapidly due to other
reactions, mainly, the reduction of water to hydrogen. If a fixed voltage
in the plateau region (for example, - 0.6V) is applied to the cathode, the
current output of the electrode can be linearly calibrated to the dissolved oxygen
(Figure 1.3b). It has to be noted that the current is
proportional not to the actual concentration but to the activity or
equivalent partial pressure of dissolved oxygen, which is often referred
to as oxygen tension. A fixed voltage between -0.6 and -0.8 V is usually
selected as the polarization voltage when using Ag/AgCl as the reference
electrode or any other EID's dissolved oxygen electrodes. Additionally
for physical and chemical correctness, partial pressure in a liquid
actually refers to the fugacity. In the pressure range relevant to the
measurements at hand, it is acceptable to equate the two values and this
allows us to restrict the following considerations to the partial
pressure. In dry, atmospheric air, the partial pressure of oxygen is
20.95% of the air pressure. This value is reduced over a water surface
because water vapor has its own vapor pressure and a corresponding partial
pressure.
Figure
1.3 (a) Current to voltage diagram at different oxygen tension; (b)
Calibration obtained at a fixed polarization voltage of –600 mV.
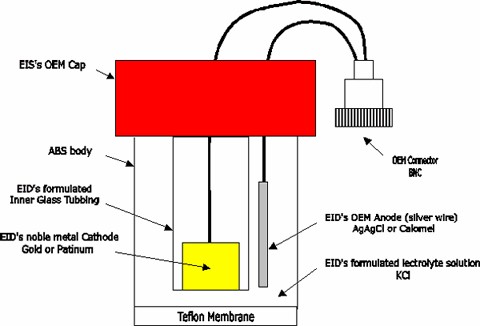
When
the cathode, the reference electrode, and the electrolyte are separated
from the measurement medium by a polymer membrane, which is permeable to
the dissolved gas but not to most of the ions and other species, and when
most of the mass transfer resistance is confined in the membrane, EID’s
electrode system can measure oxygen tension in various liquids. This is
the basic operating principle of the membrane covered polarographic
Dissolve oxygen probe (Figure 1.4).
The
basic principle underlying the electrochemical determination of oxygen
concentration is the use of membrane covered electrochemical sensors. The
main components of the sensors are the oxygen permeable membrane, the
working electrode, the electrolyte solution and a
possible reference electrode. A voltage is applied between the gold
(platinum or silver) cathode and the anode that consists of either lead or
silver (AgAgCl), and causes
the oxygen to react electrochemically. The higher the oxygen concentration
the higher the resulting electric current. The current in the sensor is
measured and, after calibration, converted into the concentration of dissolved oxygen.
If the anode is made of silver, the meter applies the required voltage (polarographic sensor). If it is made of lead, the sensor is self-polarizing, i.e. the voltage is generated in the sensor by the electrodes themselves, comparable to the process in a battery (galvanic sensor). The meter merely evaluates the current.
Figure
1.4 Basic Polarographhy electrode
EID’s
polaragraphoc dissolved oxygen electrode picture:
EID’s ELECTRODER - ABS body Dissolved Oxygen Sensor (ADO) EID’s dissolved oxygen, Probe, polaragraphic, ABS body, 12mm * 120mm, with 10K Negative Temperature Compensation Figure
1.5 Basic Polarographhy-electrode
For
our polarographic electrodes, the reaction proceeds as follows:
H202
+ 2e- -> 20H-
The
reaction tends to produce alkalinity in the medium together with a small
amount of hydrogen peroxide.
Two
principal pathways were proposed for the reduction of oxygen at the noble
metal surface. One is a 4-electron pathway where the oxygen in the bulk
diffuses to the surface of the cathode and is converted to H2O
via H2O2 (path a in Fig. 1.6). The
other is a 2-electron pathway where the intermediate H2O2 diffuses directly out of the
cathode surface into the bulk liquid (path b in Figure 1.6). The oxygen
reduction path may change depending on the surface condition of the noble
metal. This is probably the cause for time-dependent current drift of
polarographic sensors. Since the hydroxyl ions are constantly being
substituted for chloride ions as the reaction starts, KCI or NaCl has to
be used as the electrolyte. When the electrolyte is depleted of Cl-,
it has to be replenished.
2e- à (a) à
O2 à
O2 à
H2O2
Diffusion
à (b) à H2O2 Figure 1.6 alternative pathway of oxygen reduction at cathode surface
Calibration
must be carried out for dissolved oxygen measurements on a regular base.
This is because the measuring process consumes the electrolyte solution in
the sensor head, as shown by the electrode reactions presented above. The
ions of the electrolyte solution bind the released metal ions, thereby
changing the composition of the solution. The recommended calibration
period depends on the oxygen sensor used and ranges from one week for
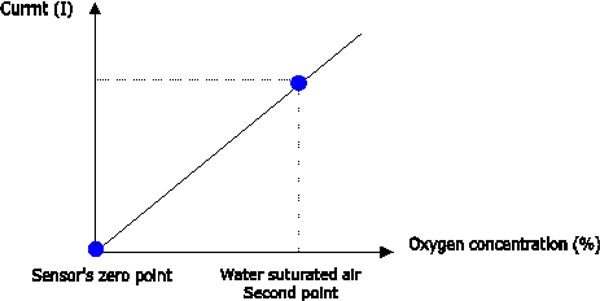
pocket instruments to 1-2 months. Each
linear calibration function is defined by at least two points. For dissolved oxygen
measurements with EID meter and/or logger, one of the
points on the line is the zero point of the sensor. At the zero point, the
sensor signal obtained in the absence of oxygen lies below the resolution
of the sensor. This point is called the zero-current point of the sensor.
The second point of the calibration line can be set as required. Its
position is based on the fact that, in a state of equilibrium, the partial
pressure of oxygen in liquid and air is equal.
Figure
1.4b Two-point calibration
The rate at which oxygen enters a dissolved oxygen probe is a function of:
As described above calibration routines for dissolved oxygen probes use a two point linear calibration where one point is at zero mg/L oxygen and the second point is at saturation or equilibrium with the atmosphere, C* . The zero measurement is not zero volts due to the conductivity of the electrolyte between the electrodes as well as any errors in the analog signal conditioning circuit. For the circuit and probe system used in the Environmental lab the zero measurement is approximately 1 mV (where approximately 200 mV corresponds to saturation levels of oxygen) and hence the zero measurement is not significant. Thus a single point calibration is used.
C* is a function of the atmospheric pressure and temperature. The functional relationship with temperature is implemented using a lookup table (based on equilibrium at atmospheric pressure) with interpolation. The effect of atmospheric pressure is implemented as shown (Equation 1 below).
P C* =------- f (T) Patm
The permeability of the membrane increases about 5% per C degree. I chose to use 25 C as the reference temperature and thus Kmembrane(Tref) has a value of 1. The following (equation 2) creates a coefficient that describes this variation.
Kmembrane(T)=Kmembrane(Tref)e0.05(T-Tref)
The slope of the linear fit (k) can be calculated after the voltage corresponding to saturation oxygen is measured (Equation 3 below).
C*cal Kmembrane C =------------------ V*cal
The slope coefficient is placed in the polynomial array.
The equation for the dissolved oxygen concentration illustrates that the predicted concentration is a function of sample temperature because Kmembrane varies with temperature. The coefficient, k, should be independent of temperature but will vary as a membrane fouls (Equation 4 below)
KV C=----------- Kmembrane
Pressure
The constituents of air have been well defined, and it is known that air contains 20.946% oxygen. Since the total pressure in the air is the sum of all of the partial pressures (Dalton’s Law), an atmospheric pressure of 760 millimeters Mercury (mmHg) in dry air will contain a partial pressure of oxygen (pO2) of approximately 159 mmHg (760 mmHg * 0.20946). Changes in atmospheric pressure will cause a directly proportional change in the partial pressure of oxygen in the air. Atmospheric pressures will vary depending upon altitude and local weather conditions. Some average pressures for varying altitudes are listed in Table 1 bellow.
The relationship between oxygen partial pressure and total atmospheric pressure should be understood and incorporated into the air calibration in order to minimize calibration error, which could be as high as 5-10% dependent upon altitude and local weather conditions. Most dissolved oxygen meters that have any sort of advanced air calibration (such as temperature compensation, which will be discussed in a later section) will be based upon an atmospheric pressure of 760 mmHg. Most tables of oxygen solubility are referenced to this value. Because of the change in oxygen partial pressure with changes in atmospheric pressure, a correction must be made when the pressure varies from this value. A simple means of incorporating pressure changes is listed in the “correction factor” shown in Table 1 bellow. The value listed is a rough multiplier, which can be used once the initial oxygen concentration is determined based upon temperature and relative humidity. A more accurate calculation for incorporating pressure will be discussed after relative humidity and temperature effects are investigated.
Some EID’s dissolved oxygen meters contain a pressure sensing device which provides compensation for pressure effects when an air calibration is performed. If you use our electrode on not-EID-meter, since most meters do not have this, it is usually necessary to note the average pressure in the local vicinity of the probe, which will be mostly altitude-based, and adjust the calibration using the simple correction factor or the more complex calculation performed later. A mercury barometer located in the immediate vicinity of the meter will give a relatively accurate measurement of the local atmospheric pressure if an older meter with no pressure sensor is used.
Table 1: Oxygen Value Corrected for Pressure (25 °C)
Relative Humidity and temperature effect and Temperature compensation
If desired the Eid's probes can be coupled with a temperature thermistor (10K Ohms) to achieve temperature compensation since Kmembrane varies with temperature.
The discussion of pressure effects were based upon atmospheric pressure with dry air (no moisture content). Whenever air contains a certain amount of moisture, the atmospheric pressure contains another source of partial pressure -- water vapor. If a comparison of the oxygen partial pressure in air with 100% relative humidity and air with 0% relative humidity is done while both are at the same atmospheric pressure, the air with 100% relative humidity will have a lower oxygen partial pressure due to the presence of the water vapor pressure (pH2O). Water vapor pressure in air varies with temperature, and is well defined. The effect of temperature on oxygen partial pressure in moist air is such that higher temperatures yield lower oxygen partial pressure, while lower temperatures yield higher pressures. Note that the effects of relative humidity and temperature can cause errors when air calibration is performed in dry air, since most of the current tables and meter temperature compensations are based on air containing 100% relative humidity. Table 2 bellow shows both the oxygen concentration, which is linear with the partial pressure of oxygen, that would be present at 100% relative humidity and 0% relative humidity. The values only differ by a few percent in ambient air conditions, and thus is generally ignored. Most dissolved oxygen meters have temperature compensation for air at 100% relative humidity, and no manual correction is necessary. However, many older meters do not have temperature compensation included, and therefore this calculation must be done manually. If temperature is not compensated for in the calibration, the error can be as much as 20 to 30 % for every 10 degrees difference from 25 °C, and therefore temperature compensation is standard on most dissolved oxygen meters today. Since the effects of relative humidity is minimal at all but the highest temperatures, no current dissolved oxygen meters incorporate any kind of relative humidity sensing device. In order to ensure an accurate temperature and current reading, the probe must be exposed to the air for enough time to allow thermal equilibrium to occur. There are often significant temperature differences between the process water and the ambient air. Larger temperature gradients between the two necessitate additional time for thermal equilibrium to take place. For instance, a 20 °C difference between ambient air and process water can cause a calibration delay of about 30 minutes in many probes for the probe to fully equilibrate to ambient temperature. Since most temperature gradients will not be this large, allowing approximately 15 minutes is usually a safe assumption. It is common for users to calibrate the unit before the dissolved oxygen meter is reading the stabilized temperature and current value, which can cause significant error since a difference of even 5 °C from actual can cause the reading be off by 5 to 10%. It is often useful to have a calibrated temperature sensor, accurate to 1 °C or better, at the calibration location to know when the probe temperature is reading the correct ambient air temperature. It is useful to have an equation which can be used to determine oxygen concentrations in air based upon temperature, relative humidity, and pressure. Since the full equation is quite lengthy and complex, two easier versions are presented to the user, along with Table 2 bellow, to determine the correct oxygen concentration in air. Equation 5 bellow should be used with air with 100% relative humidity, and Equation 6 should be used for air with 0% relative humidity.
Equation 5 (100% Relative Humidity): OS = (OS’) * (P - p) / (760 - p) where:
OS = Oxygen solubility at barometric pressure of interest OS’ = Oxygen in saturation at one atmosphere (760 mmHg) at a given temperature P = Barometric pressure of interest p = Vapor pressure of water at the temperature of interest Example 1:
The user wishes to calibrate a dissolved oxygen probe in air at an altitude of 3500 feet. The temperature is 30 °C, and the relative humidity is 100%. At an altitude of 3500 feet, the atmosphere pressure will usually be about 668 mmHg (Table 1 above). The sample temperature is 30 °C, and the relative humidity is 100%. From water vapor pressure tables, the water vapor pressure at 30 °C is 31.8 mmHg. The oxygen saturation level at 760 mmHg and 30 °C is 7.54 ppm (Table 2 bellow). Substituting these values in the above (equation 5) gives the following:
OS = (7.54) * (668 - 31.8) / (760 - 31.8) = 6.59 ppm
Example 2:
Assume the same conditions as in example 1, but with a relative humidity of 0%. In this case, the value used for the oxygen saturation level would be 7.87 (Table 2 bellow), not 7.54. The calculation will change since there will be no water vapor pressure. Equation 6 (0% Relative Humidity): OS = (OS’) * (P) / (760 mmHg) Substituting the above values into the equation yields the following:
OS = 7.87 * (668) / (760) = 6.92 ppm Note: that the multiplier of (668) / (760) is actually the simplified correction factor listed in Table 1 above for an altitude of 3500 feet (0.88). Table 3 bellow lists calibration values for varying temperatures pressures at relative humidity levels of 100%.
Table 2 above: Dissolved Oxygen Solubility vs. Temperature
Table
3 above: Oxygen concentration (ppm) for varying pressures (mmHg) and
Calibration
in air saturated with water vapor:
This
requirement is met over large water surfaces, such as lakes or the sludge
activation basin of a wastewater treatment plant. Note: EID’s offers special air calibration vessels for laboratory measurements.
Calibration
in air saturated water:
The
water is aerated until the partial pressure of the oxygen in the water is
the same as in the air. This method is accompanied by some inherent risks:
As
you can see from the above list, is clearly preferable to calibration in
air-saturated water.
Relative
slope limitation, to evaluate the sensor function despite it, there are
three characteristic measuring points in addition to a visual test. In
the visual test, the gold cathode is examined visually. If it has lost its
gold or platinum color and is coated with lead or silver, the sensor will
yield values that are too high and will generally no longer be
zero-current-free. This can be corrected by regenerating the oxygen sensor
as described in the operating manual. The gold cathode may only be
polished with a moist special abrasive film using a circular motion with
little pressure. It is imperative that only this special film be used
since a scratched and unpolished electrode surface can harm the sensor and
impair its accuracy. Attention:
Anodes of lead or silver cannot be polished at all. One
subjective, visual examination of the sensor can be supplemented by a more
comprehensive test, an evaluation at three specific measuring points: in
air saturated with water vapor (1), in air saturated water (2) and in
oxygen free water (3). 1.
Test in air saturated with water vapor: The
sensor should obtain a reading between 100 and 104% oxygen saturation in
water-saturated air. If the values lie above this range, the membrane was
probably wet during calibration; perhaps there is too much water in the
calibration vessel. A value above 100% saturation is due to the differing
viscosity of water and air as well as to the surface tension of water. To
put it simply, it is easier for oxygen molecules in the air to permeate
the membrane than for those in the water to dissolved oxygen so. In the measuring mode,
which is the mode in which the test takes place, calculations are based on
a liquid sample and this results in a saturation level over 100%. 2.
Test in air saturated water: After
calibration, the value in air-saturated water should lie between 97 and
102% saturation. The theoretical value is 100% but is difficult to
reproduce. This relatively large tolerance is due not to the sensor but to
the saturation procedure. This is also the reason why EID successfully
sought an alternative to the conventional calibration procedure in
air-saturated water. If the sensor dissolved oxygen not display a reading within this
tolerance range, it should be sent back to the manufacturer for tests. 3.
Test using zero solution: This
is to test the zero current point of the sensor. When the oxygen content
is 0 mg, the maximum reading of the sensor should not exceed the
resolution of the meter (1 digit). This test is carried out using sodium
sulfite solution. Sulfite reacts with the dissolved oxygen to form
sulfate, binding the oxygen dissolved in the water. Preparation
of the solution: Dissolve a teaspoon of sodium sulfite in
100 ml tap water. The solution will be oxygen free after it has stood
undisturbed for 15 minutes. It must remain undisturbed to prevent oxygen
in the surrounding air from re entering the solution. One minute after
submerging paleographic sensors (EID-E-ADO–A001,
EID-E-ADO-A002, etc.)
Into the solution, the meter should display a maximum reading of 2%; after
15 minutes, the maximum reading should be 0.4%. If not, the sensor is no
longer zero current free and must be cleaned or sent to the manufacturer
for tests. After the test, the sensor should be rinsed thoroughly with
distilled water to remove any remaining traces of sodium sulfite solution.
Galvanic
sensors with lead counter may be submersed for no more than 3 minutes.
Subsequently, they must also be rinsed thoroughly with distilled water.
Cleaning of the sensors is extremely important to prevent toxification and
lasting damage.
Measurement
and analytical quality assurance:
Measurement
of the oxygen concentration is now quite easy to carry out. The sensor is
submersed in the liquid to be investigated and the measured value is read
from the display. In principle, this is all there is to it, but
nevertheless a few important points should be observed and among those is
the proper maintenance of the sensors.
The
component of the sensor that is sensitive to contamination is the
membrane. Contamination results in lower readings when measuring or lesser
slopes when calibrating because a portion of the membrane surface is not
available for the diffusion of oxygen. The attempt to compensate for the
contamination by adjusting the instrument does not agree with the water
principle. It is preferable to clean the membrane. Acetic or citric acid
with a concentration of 5--10% (percent in weight!) is used for calcium
and iron oxide deposits and warm (<50C) household detergent is used for
fats and oils. Avoid
strong mechanical treatment of the membrane during all cleaning activities
because its thickness is on the order of m and it is easily destroyed. It
is best to use a soft paper towel. Dissolved oxygen not clean the sensor in an
ultrasound bath as this may cause the coating of the anodes to peel off.
Regeneration
of the sensor becomes necessary when the function responds or when the
slope (S) < 0.6 has decreased markedly when calibrating. Basically,
regeneration is required when the electrolyte solution is depleted, when
the gold cathode has become coated with lead or silver, when the reference
electrode is to xified or when the membrane is damaged or contaminated. It
consists of exchanging the electrolyte solution, cleaning the electrodes
and exchanging the membrane head. It
is important to follow the operating manual exactly! Mistakes are then
easily avoided. The
following points should be emphasized:
Please
note: The spacing lattice is clearly visible when the membrane head
is held up against the light. The
result of an oxygen measurement can be documented in several ways:
Polarization
periods (startup periods) prior to measurement:
If
the sensor was disconnected from the meter, an appropriate polarization
period must elapse after the polarographic sensors are reconnected
(gold-silver electrode system) before the start of measurements. Please be
advised that this does not apply to galvanic sensors (gold-lead electrode
system) because they are self polarizing and can be used immediately.
The
approach flow to the sensor membrane must be continuous for oxygen
measurements to be correct. The diffusion of the oxygen molecules in the
sensor head creates an oxygen poor zone that simulates a reduced
concentration of oxygen. The concentration at the membrane must always be
the equal to the concentration in the remainder of the sample. This
condition can be met by stirring the sample or moving the sensor in the
sample. EID
offers special stirring attachments that rotate like little turbine blades
and continuously supply the membrane with fresh sample. They are driven by
an alternating electromagnetic field that is generated by the base of the
stirrer.
The
major advantage of this unit is the size of the attachment. It has the
same diameter as the sensor and is mounted on the sensor head. This
simplifies measuring in sample bottles such as bottles for BOD
measurements. The
EID-E-ADO–A001 sensor is specially designed for BOD measurements. The
sensor shaft contains a propeller similar to a marine screw propeller to
maintain a continuous approach flow at the membrane. The stirring effect
is sufficiently large to homogenize the sample in addition to generating
the approach flow. If
agitators or magnetic stirrers are used, the possible formation of eddies
must be taken into account. The oxygen sensor may not be positioned in the
eddy because air at the sensor head Approach
flow membrane may falsify readings. This can be prevented by lowering the
stirring frequency or positioning the sensor away from the eddy. When
the sensor is installed in pipelines, the sample flows past the sensor
head, providing a sufficient approach flow. EID offers stationary
measuring systems with special installation assemblies for pipes. Alternatively,
the sensor itself can be moved in the medium being investigated, e.g. by
stirring the sensor in a beaker or by swinging it back and forth in a
lake. For measurements at great depths, depth armatures for water depths
up to 100m are available. It
is important that stirring does not falsify the measured values. This is
likely to happen when the investigated sample is supersaturated or under
saturated with oxygen and oxygen can be expelled from or stirred into the
sample. Super
saturation with oxygen, for example, can be observed in the summer in
stagnant waters when luxuriating algae produce oxygen by photosynthesis.
An example of under saturation with oxygen is the BOD determination in
which bacteria lower the oxygen concentration in bottles through
respiration. For this reason, the volume of the sample is important. A
measurement taken in a lake or an activation basin is uncritical because
of the enormous quantity of the sample. In an open beaker, however,
stirring can easily alter the oxygen concentration.
The
temperature dependent Bunsen absorption coefficient changes when
substances are dissolved in the water. This effect is accounted for by
entering the salinity. The salinity can be determined using a conductivity
meter and corresponds to the salt content of seawater in g/kg. The
standard recommends the use of this function for other waters since the
deviation is minimal (<2%).
Influence
of interfering gases:
The
membrane is also permeable to gases other than oxygen. Nitrogen does not
react and is, therefore, irrelevant. The high pH value of the electrolyte
solution protects the measurement from the interfering influence of
ammonia. Carbon dioxide, on the other hand, is problematic. The buffering
capability of the electrolyte solution is sufficient for short-term
exposure; during long-term exposure, however, carbon dioxide shifts the pH
value into the acidic range and leads to increased values. Polarographic
sensors can better regenerate the buffer capability than galvanic sensors
because they generate an excessive number of hydroxide ions during the
electrode reactions. The buffer capacity of the electrolyte solution in
the sensor is insufficient for samples with a high carbon dioxide content
(e.g. beer, sparkling wine or soft drinks). The pH shifts into the acidic
range and the meter shows higher than normal readings. Hydrogen sulfide
presents the greatest danger for oxygen sensors because the sulfide-ions
generated by the neutralization reaction toxify the counter electrode. The
sensors can withstand small amounts, but continuous exposure markedly
shortens their lifetime. Hydrogen
sulfide has the smell of rotten eggs and is easily perceptible at very
small concentrations, eliminating the need for complex measurements.
In order to determine the concentration of dissolved oxygen in non-aqueous liquids, the appropriate solubility function must be known. High performance dissolved oxygen meters and logger from EID have stored software programs that make this type of determination feasible. If the solubility function is known, oxygen measurements can be carried out similarly to measurements in water.
Checking the oxygen meter and or logger:
The METER-D0 (meter) or PROBER-DO (data logger) are checked using simulators. The simulators are connected to the instrument in place of the sensor. They generate defined current signals that the instrument must display correctly. If the readings lie outside the tolerance indicated by the certificate, the instrument must be sent to the manufacturer for servicing.
A.
Foods and Beverages C.
Fish Farming - Aquaculture F. Sea cages
K. Measuring biochemical oxygen demand
The BOD test requires a commitment of five (5) days from initial sample collection to the end of the analysis. During this time, samples are initially seeded with microorganisms and supplied with a carbon nutrient source of glucose-glutamic acid. The sample is then introduced to an environment suitable for bacterial growth at reproducible temperatures, nutrient sources and light within a 20C incubator such that oxygen will be consumed. Quality controls, standards and dilutions are also run for accuracy and precision. Determination of the dissolved oxygen within the samples can be determined through titration. The difference in initial DO readings (prior to incubation) and final DO readings (after a five (5) day incubation period) predicts the BOD of the sample. A suitable detection limit as per environmental quality control is 1 mg/l. BOD calculations
Steps to calculate Biochemical Oxygen Demand (BOD). They and are based on the addition of a nutrient source (carbon - glucose - glutamic acid) and no nutrient source.
1. The BOD of the blanks (no nutrient source) = DOFinal - DOInitial
2. The BOD of the nutrient added samples = (DOFinal - DOInitial) time dilution factor per 300ml
* 300 ml is based on the volume contained in BOD bottles
The BOD of the sample and standards are calculated by subtracting the final DO from the initial DO and multiplying this factor by the dilution factor. The final value is determined by subtracting out the BOD for the blank from the BOD that has been nutrient enriched.
Preparations:
All
practical experiments should be carried out in a suitable laboratory to
guarantee working safety. This is a general recommendation. In the field
of oxygen measurement, the cleaning solutions and the electrolyte
solutions may contain caustic substances. A possible danger therefore
exists when regenerating the sensor. Safety
instructions. General rules of conduct when handling chemical substances When
working at a work place at which chemicals are handled, the following
rules must be observed: 1.
Follow the instructions on the chemical bottles 2.
Always wear protective garments (goggles, gloves...) 3.
Never point open containers towards other persons 4.
Do not eat, drink or smoke 5.
Ensure the satisfactory disposal of chemicals 6.
Carefully remove or clean up any spilled chemicals 7.
Contact specialist personnel if any serious problems arise We
provide these short instructions in the hope that they lead to successful
and safe practical research and studies. Safety
data sheets are available for the cleaning and electrolyte solutions. The
user must have one of these data sheets. Because they are fairly
comprehensive, they cannot be included in every delivery. The manufacturer
will, however, provide them on request. The
following equipment and facilities must be present when you are measuring
dissolved oxygen:
|